Alberti Lab
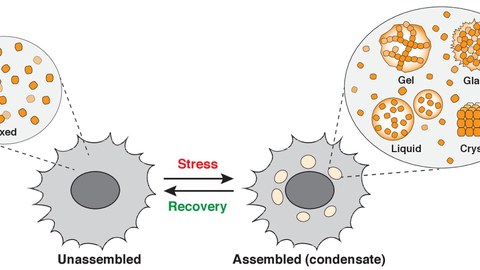
We still know very little about the organization of the cytoplasm and the role of membraneless compartments in regulating the physiology of cells. My research group aims to elucidate the molecular principles underlying the organization of the cytoplasm. We are particularly interested in understanding how the cytoplasm changes upon environmental stress. Stressed cells have to adapt their physiology and metabolism to the new conditions. These adaptations are often mediated by alterations in the structure and organization of the cytoplasm. We aim to understand how these alterations promote organismal survival but we are also very interested in how they cause disease.
 © vipra gen (Pixabay)
© vipra gen (Pixabay)
Our research
The cytoplasm is a mysterious jelly-like substance that sustains the biochemical reactions that are essential for life. How the cytoplasm organizes itself is one of the big remaining questions in biology. We use cell biological, biochemical, biophysical and genetic approaches and diverse model systems, such as yeast, Dictyostelium, and mammalian cells, to elucidate the molecular principles underlying the organization of the cytoplasm. We are particularly interested in understanding how the cytoplasm reorganizes itself upon environmental perturbations and stress. Stressed cells undergo changes on many levels to alter their physiology and metabolism; we are beginning to understand that many of these changes result from alterations in the structure and organization of the cytoplasm.